Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124

Water for Injection (WFI) plays a vital role in pharmaceutical manufacturing, serving as a critical component in the production of various pharmaceutical products.
It is a specialized form of water that meets stringent quality standards, ensuring its suitability for use in the manufacturing of injectable drugs, sterile preparations, and medical devices.
The quality and purity of WFI are paramount, as it directly impacts the safety and efficacy of the final pharmaceutical products.
In pharmaceutical manufacturing, WFI is used in a range of applications, including the formulation of parenteral solutions, reconstitution of powdered medications, and as a solvent for active pharmaceutical ingredients.
As WFI comes into direct contact with the human body through injections, infusions, or inhalation, it must be free from impurities and contaminants to prevent any adverse effects on patients.
The production of WFI requires specialized processes and equipment to meet the stringent purity requirements.
Various methods are employed, such as Multi Column Distillation (MCD), Vapor Compression Distillation (VCD), and Cold WFI process, each with its own advantages and considerations. It is crucial for pharmaceutical manufacturers to understand these processes and their implications to ensure a consistent supply of high-quality WFI.
In this blog, we will delve into the comparison between the Multi Column Distillation (MCD) plant, the Vapor Compression Distillation (VCD) plant, and the Cold WFI process.
We will explore their characteristics, benefits, limitations, and alignment with regulatory requirements.
By gaining insights into these processes, we can better appreciate the importance of WFI in pharmaceutical manufacturing and the considerations involved in selecting the appropriate method for its production.
Let us first understand the overall process of producing Water for Injections with three different technologies.
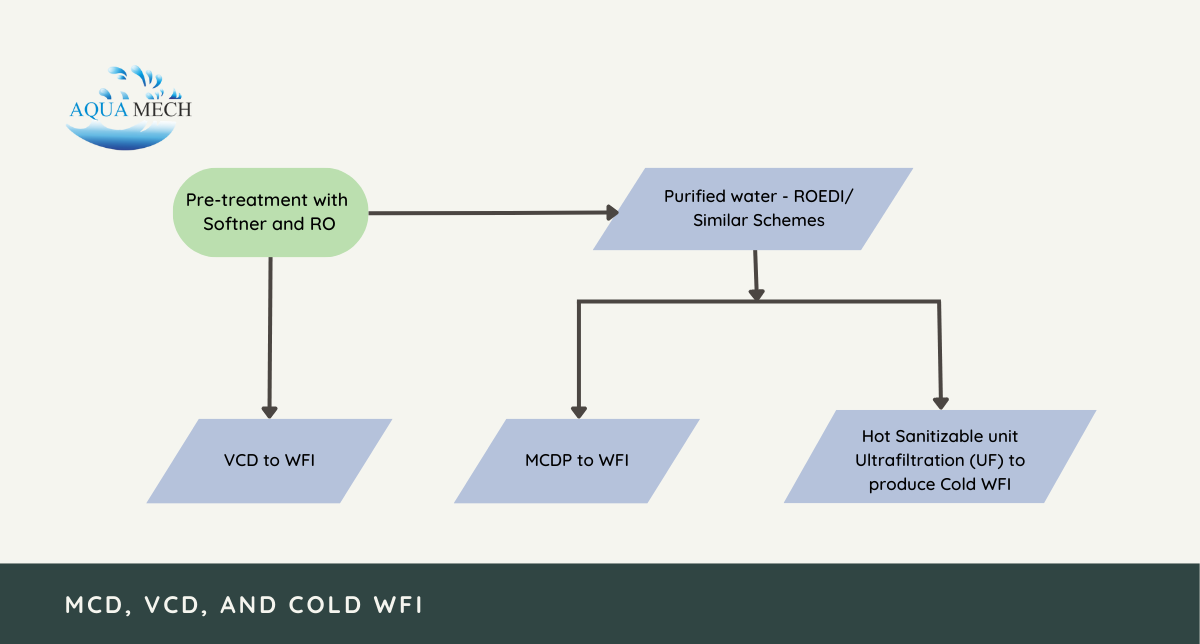
The process of producing Water for Injection (WFI) involves multiple stages of pre-treatment and purification to adhere to stringent pharmaceutical purity standards.
Initially, the pre-treatment involves the utilization of water softeners and reverse osmosis to eliminate impurities from the initial water source.
Subsequently, this treated water is directed to a Vapor Compression Distillation process, which is employed to generate water for injection if this particular technology is used in the production.
In case the production involves the application of Multi-Column Distillation Plant (MCDP) technology, the purified water undergoes an additional step. It is sent to either ROEDI or similar schemes before being fed into the MCDP to produce water for injection.
For the Cold WFI process, the purified water obtained from ROEDI or similar schemes undergo a hot sanitizable Ultrafiltration (UF) process. This step is crucial as it effectively removes any remaining contaminants and reduces the microbial load present in the water.
First, let’s explore each of the three processes in depth, and then we will proceed to compare the technologies to determine which one is superior and the reasons behind it.
The Multi Column Distillation Plant (MCDP) is an energy-efficient industrial equipment that produces high-purity water or liquids through distillation.
It utilizes multiple distillation columns arranged in a cascading fashion to achieve progressive purification by reusing energy from the previous column.
They can produce water with a purity exceeding 99.9%, suitable for pharmaceuticals, power generation, and more. MCDPs are sophisticated, energy-efficient systems used in industries requiring high-purity liquids.
The Multi Column Distillation Process (MCDP) originated from the development of distillation techniques for water purification. It emerged in the mid-20th century as a way to enhance the efficiency of large-scale water purification.
The MCD process works as follows:
Advantages of MCDP:
Limitations of MCDP:
The Multi Column Distillation Process (MCDP) has been accepted by United States Pharmacopeia (USP) and Japanese Pharmacopoeia (JP), European Pharmacopeia (EP), and World Health Organization (WHO), and many other regulatory authorities for producing WFI.
In addition to these regulatory authorities, MCDP is also accepted by regulatory authorities in other countries, such as India, Australia, the United Kingdom, China, and Brazil.

Vapor Compression Distillation (VCDP) is an alternative method used for the production of Water for Injection (WFI) in the pharmaceutical industry. It offers a different approach to achieving water purity compared to Multi Column Distillation (MCDP).
It is a process that uses a compressor to increase the pressure of the water vapor, which then condenses to produce WFI. This process is more energy-efficient than multiple-effect distillation, but it can be more expensive to install.
The VCD process works as follows:
Understanding the VCD process the advantages and limitations of this method is essential for pharmaceutical manufacturers considering its implementation.
Advantages of VCDP:
Limitations of VCD:
The Vapour Compression Distillation Process (VCD) has been accepted by United States Pharmacopeia (USP) and Japanese Pharmacopoeia (JP), European Pharmacopeia (EP), and World Health Organization (WHO), and many other regulatory authorities for producing WFI.
In addition to these regulatory authorities, VCD is also accepted by regulatory authorities in other countries, such as India, Australia, the United Kingdom, China, and Brazil.
The Cold Water for Injection (WFI) process is an alternative method for producing high-quality water for injection in the pharmaceutical industry.
Unlike traditional distillation methods such as Multi Column Distillation (MCD) and Vapor Compression Distillation (VCD), the Cold WFI process relies on other membrane-based and non-distillation purification techniques to meet the required standards of WFI.
The cold WFI process works as follows:
1. Pretreatment: The first step is to pretreat the feed water to remove any contaminants that could interfere with the subsequent purification steps.
2. ROEDI: Next, the feed water is directed to a process like Reverse Osmosis Electrodionization (ROEDI) or similar methods.
3. Ultrafiltration (UF): UF employs membrane filters with microscopic pore sizes (6k daltons or MWCO) capable of removing particles as small as protein macromolecules. These membranes and complete piping is hot water sanitizable with hot water at 80C. This membrane filtration removes endotoxins/pyrogens and usually has a log 6 reduction of microbial counts.
With all other parameters like pH, TOC, Conductivity, and Microbial load of <100 cfu/ml achieved till ROEDI outlet then Hot water sanitizable UF ensures that there is a further reduction of microbial contamination and also removal of endotoxins/pyrogens as per the guidelines of regulatory authorities for Water for Injection.
The cold WFI process is a critical step in the production of sterile pharmaceutical products. By following the proper procedures, manufacturers can ensure that the water used in their products is of the highest quality and meets all regulatory requirements.
Advantages of the Cold WFI:
Challenges of the Cold WFI:
The production of WFI is subject to strict regulatory guidelines and requirements to ensure its safety, purity, and quality.
The European Pharmacopoeia (EP) updated its monograph for Water for Injections (WFI) in April 2017. This update marked a pivotal moment for WFI manufacturers in Europe, as it granted them the freedom to choose between two methods of production: traditional distillation systems (hot WFI) and modern Reverse Osmosis (RO) based membrane systems (cold WFI).
Prior to this update, only distillation was allowed, but now, in line with other renowned pharmacopeias like the United States Pharmacopeia (USP), Japanese Pharmacopoeia (JP), and World Health Organization (WHO), Europe embraced non-distillation methods as well.
This reform had a profound impact on the global landscape of WFI production. Gradually, numerous countries worldwide, including Australia, New Zealand, Brazil, China, and India, followed suit and began recognizing cold WFI as a preferred method for producing water for injection.
Alignment of Each Process with Regulatory Standards:
It is crucial for pharmaceutical manufacturers to carefully design and operate their WFI production processes in accordance with regulatory guidelines.
This includes implementing appropriate quality management systems, conducting regular testing and monitoring, and maintaining documentation to demonstrate compliance with regulatory standards.
By adhering to regulatory guidelines, manufacturers can ensure the production of WFI that meets the required quality, purity, and safety standards, thereby contributing to the overall quality and efficacy of pharmaceutical products.

Let’s delve into the comparison of Cold Water for Injection (Cold WFI), Multi-Column Distillation Plants (MCDPs), and Vapor Compression Distillation Plants (VCDPs).
Each of these technologies plays a crucial role in the production of Water for Injection (WFI) in the pharmaceutical industry.
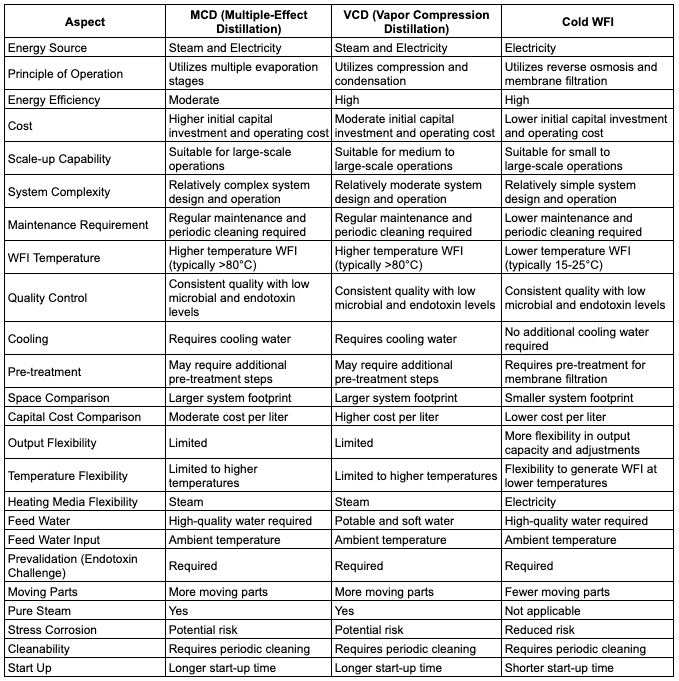
Cold WFI stands out as the winner in several aspects:
Cold Water for Injection (WFI) emerges as the winning choice for pharmaceutical manufacturers seeking a highly efficient and cost-effective method for producing high-quality water.
With its lower capital costs, excellent energy efficiency, ease of scalability, and advanced microbial control, Cold WFI stands out as the ideal solution. Moreover, its reduced environmental impact through water conservation makes it an environmentally friendly option.
For expert guidance and implementation, don’t hesitate to reach out to our Design Head, Navdeep Singh Sethi or you may contact Aquamech today at hipurity@aquamech.co.in to elevate your pharmaceutical manufacturing with Cold WFI.